
This World Malaria Day, we take a look at five challenges we are helping to tackle with genomic surveillance, by constantly monitoring how these deadly parasites – and the mosquitoes that spread them – mutate and evolve across the world and over time. The Genomic Surveillance Unit (GSU) at the Wellcome Sanger Institute curates large-scale data resources to better understand and track these challenges.
The challenge: Since the middle of last century, a series of promising drugs have been rolled out globally to treat malaria, only for parasites to evolve resistance that makes the drugs ineffective. In the early 2000s, the World Health Organization (WHO) recommended a slightly different approach: artemisinin-based combination therapy (ACT). There are different combinations of ACTs, each involving a drug based on artemisinin plus one or more different ‘partner drugs’.
However, artemisinin partial resistance has emerged in several locations, meaning the drugs take longer to clear malaria parasites from the blood. The concern now is that if resistance to the partner drugs also develops – as it already has with several compounds – then ACTs will no longer be an effective treatment.
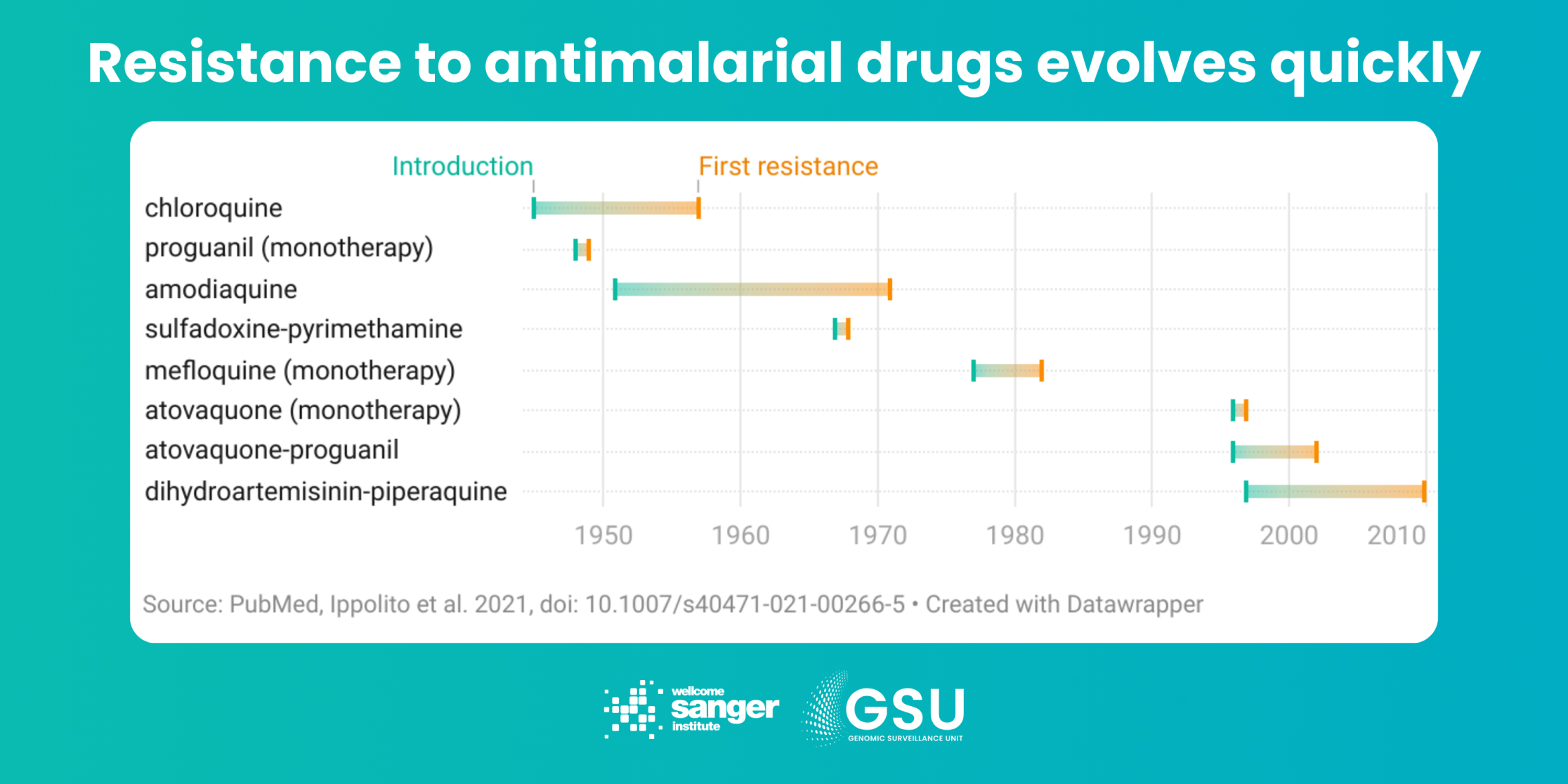
How genomic surveillance can help: Artemisinins are not the first class of antimalarial drugs to face parasite resistance. However, this is the first time we have had a warning by discovering its molecular marker – a specific region of DNA known as PfKelch13 – before the problem became widespread. Using targeted techniques such as amplicon sequencing, genomic surveillance can monitor parasites for mutations in PfKelch13, and inform public health authorities on artemisinin resistance spreading in their area. The Pf7 data resource, published in 2023, shows which drugs are likely to work on more than 20,000 samples from 97 locations around the world.
On top of this, whole-genome sequencing (WGS) can be used more broadly to spot changes across parasites’ genomes, to detect previously unknown mutations responsible for other threats, such as resistance to partner drugs.
The challenge: Insecticides in bednets and indoor sprays have helped make major gains against malaria this century. But the Anopheles mosquitoes that carry malaria parasites are evolving resistance to these too, in particular a widely used group of chemicals called pyrethroids. New mosquito-killing products are coming on the market, such as bednets using a mixture of insecticides, and sprays using pyrethroid alternatives, such as neonicotinoids and chlorfenapyr. But these take a lot of time and money to develop and test. And, given time, the insects will likely evolve resistance to these too.
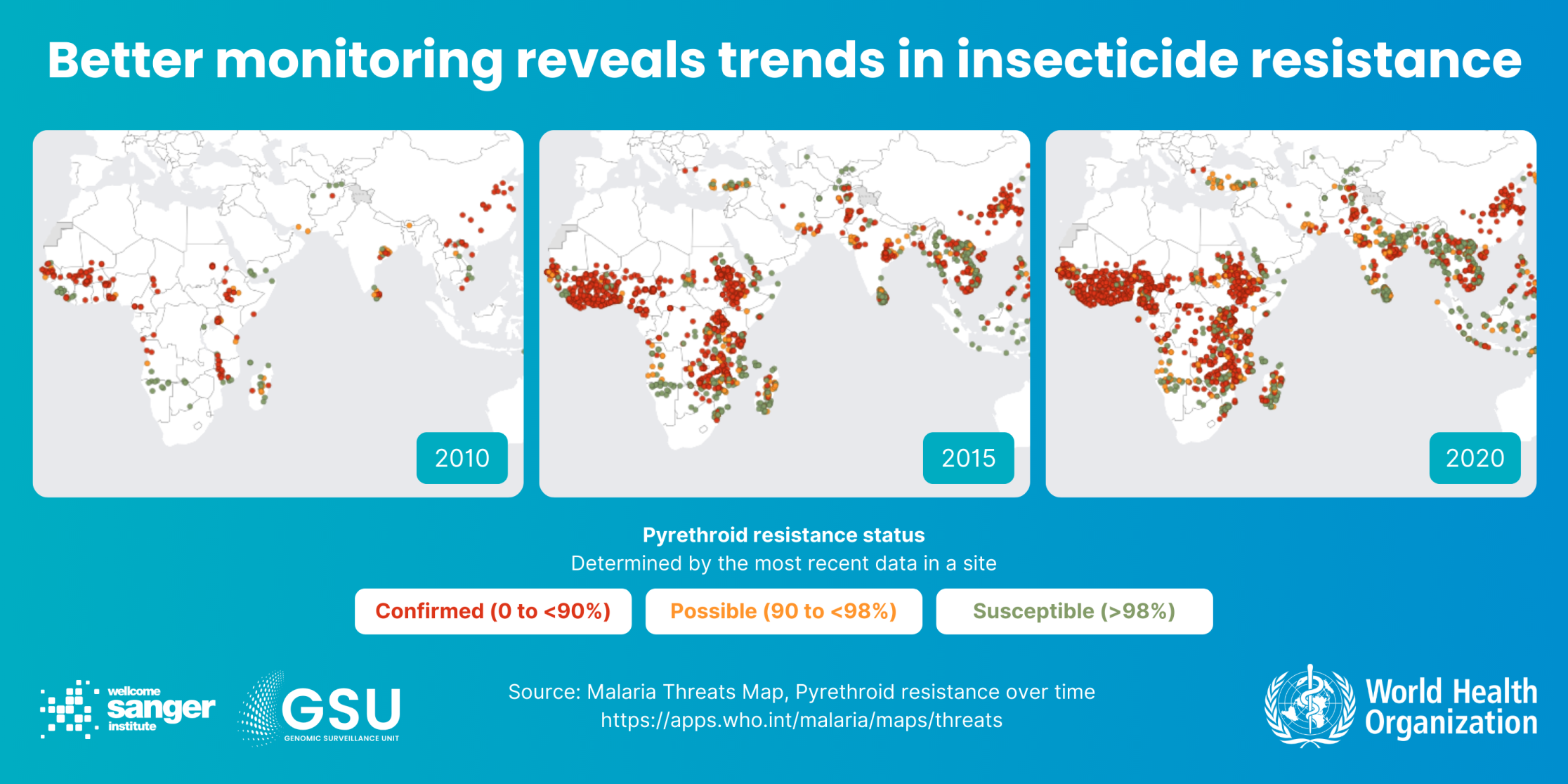
How genomic surveillance can help: We already know many mutations which cause insecticide resistance. But it’s a complex picture. Mosquitoes have evolved several ways of surviving the chemicals, including metabolising them with enzymes and stopping them binding to receptors. Genomic surveillance monitors known genetic insecticide resistance markers, and allows us to spot patterns that reveal new resistant variants as they evolve. By conducting studies over time, and by collecting additional data – for example, on mosquito ecology or behaviour – we can also understand what drives these genetic changes.
The GSU contributes to the Malaria Vector Genome Observatory, building cloud-native, easy-to-use analysis tools to make the most of what has become the world’s largest genomic data resource on a eukaryotic organism.
Ultimately, this informs how public health authorities use insecticide products. For example, should expensive new nets be distributed in a region, or has pyrethroid resistance not developed yet and cheaper nets will have a similar effect? These real-world decisions are particularly key when resources are limited.
The challenge: The introduction of disposable rapid diagnostic tests (RDTs) – similar to the lateral-flow tests later used for COVID-19 – was a cheap and versatile breakthrough for diagnosing malaria. They help decide treatment and prevent the unnecessary use of drugs, which can drive parasite resistance. These tests contain antibodies that detect the histidine-rich protein 2 (HRP2) and/or a related protein HRP3. However, strains of the Plasmodium falciparum parasite have evolved deletions of the genes encoding these proteins, pfhrp2 and pfhrp3, which means they avoid detection by the tests. In some countries, the prevalence of these deletion mutants is high enough to undermine RDT results. The tests can’t reliably tell the difference between a blood sample that has no malaria parasites in it and one that has deletion mutant parasites.
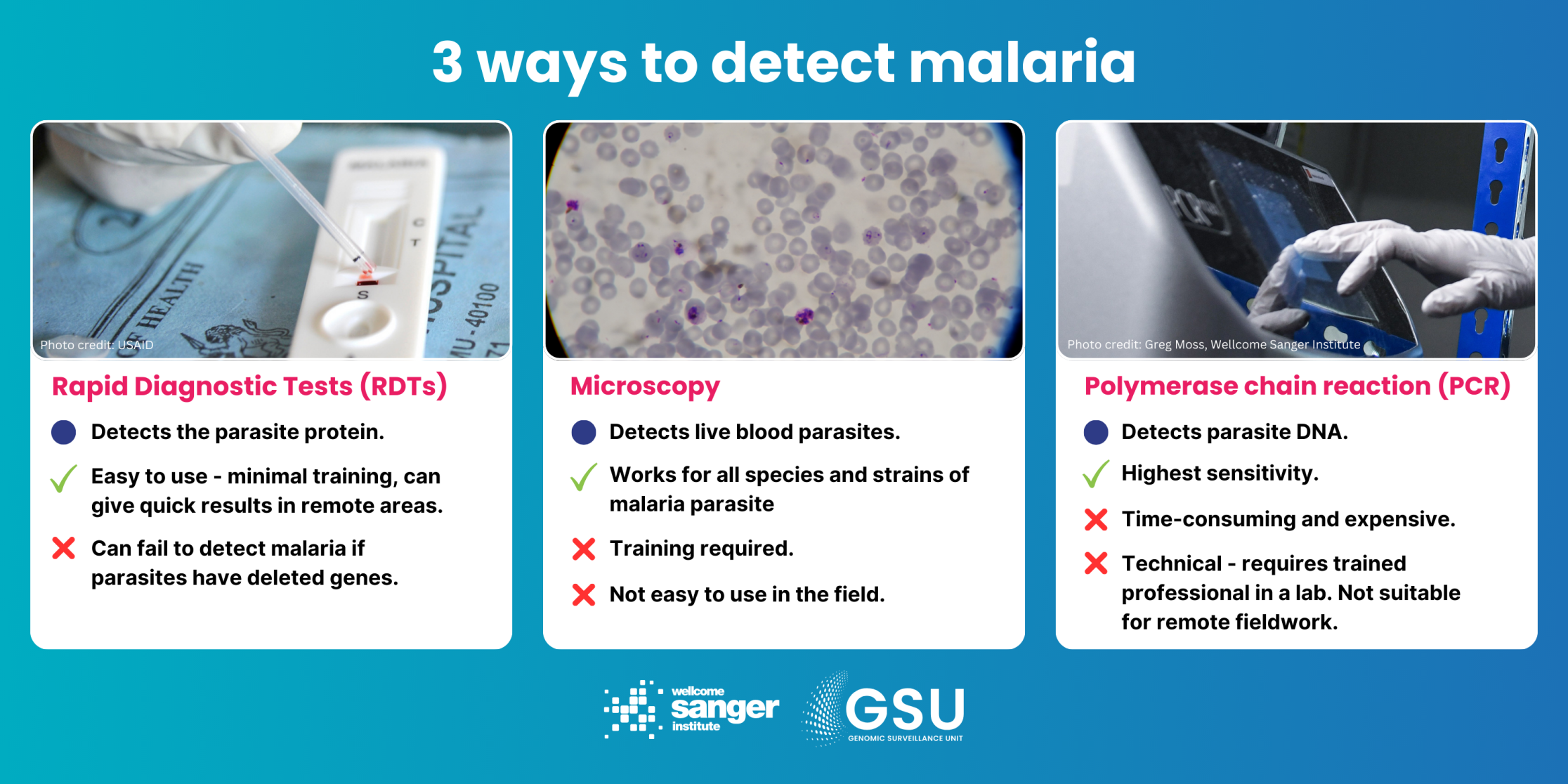
How genomic surveillance can help: By sequencing the genomes of many parasites in many countries – the MalariaGEN Pf7 database now has over 20,000 whole genome sequences from 33 countries – we can investigate the levels of parasites with the hrp gene deletion in different locations, and gauge whether the problem is getting worse. Public health authorities then know whether to trust RDT diagnoses, or whether to consider switching to other tests. Genomic surveillance can also help us to understand whether gene deletions are spreading between locations or popping up independently.
The challenge: Anopheles mosquitoes and the Plasmodium parasites they carry are highly mobile organisms, with our human activities helping both spread far and wide. Partial artemisinin resistance and RDT gene deletions in parasites (see above) were first observed in Southeast Asia and the Peruvian Amazon respectively – both are now causing concern in Africa. Genomic surveillance can show how these variants spread across continents, whether they have spread globally from region to region, or whether they are evolving independently.
The standout example of mosquito mobility is a species from South Asia, Anopheles stephensi, which arrived in Djibouti in 2012 and has now been confirmed in seven African countries. Unlike its African relatives, this Anopheline is a day-biter, which means bednets won’t work. It is also much more at home in urban settings, readily breeding in often tiny and temporary water sources, such as man-made containers. Its arrival has already resulted in malaria outbreaks in African cities which seldom saw malaria before.
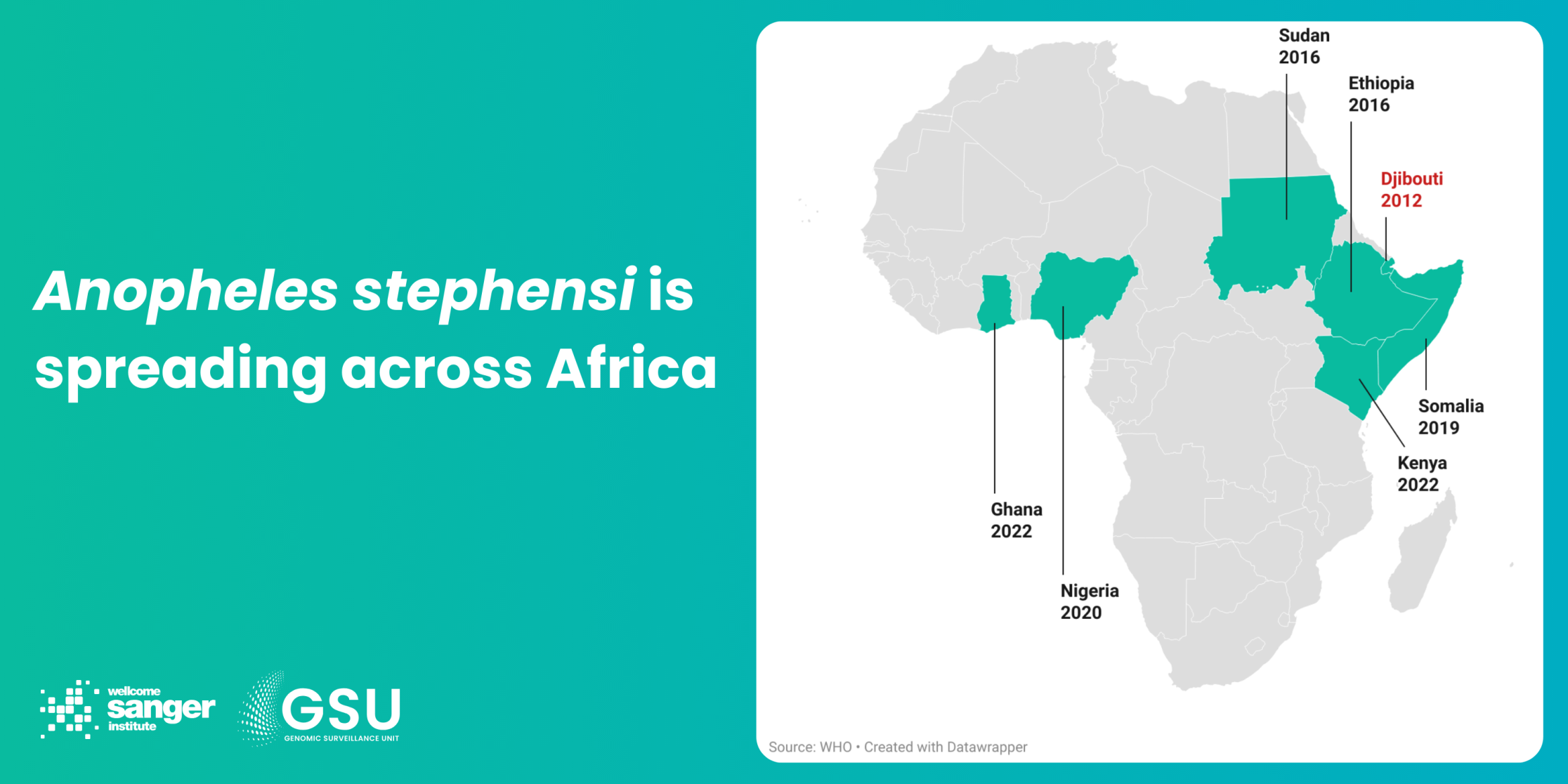
How genomic surveillance can help: In cases such as An. stephensi, genomic surveillance has the potential to reliably track the migration of the species in near-real time. This might provide a crucial early warning to authorities to start checking urban water sources for breeding sites. Genomics also allows us to see how closely populations are related, both for parasites and mosquitoes – whether we’re looking at one big interconnected population over a large area, or separate populations that haven’t mixed for a long time. We can then predict the likelihood of them coming into contact, reproducing, and spreading traits such as resistance over a few generations.
The challenge: Since 2021, the WHO has recommended two new malaria vaccines: RTS,S and R21. This is a major breakthrough, the first successful vaccines against a eukaryotic species, rather than a virus or bacterium. Trials have shown both to be effective and safe, and RTS,S has already reached 2 million children in Africa. This is a good news story 60 years in the making. But as malaria has shown in the past, our ultimate hurdle against this disease can be complacency.
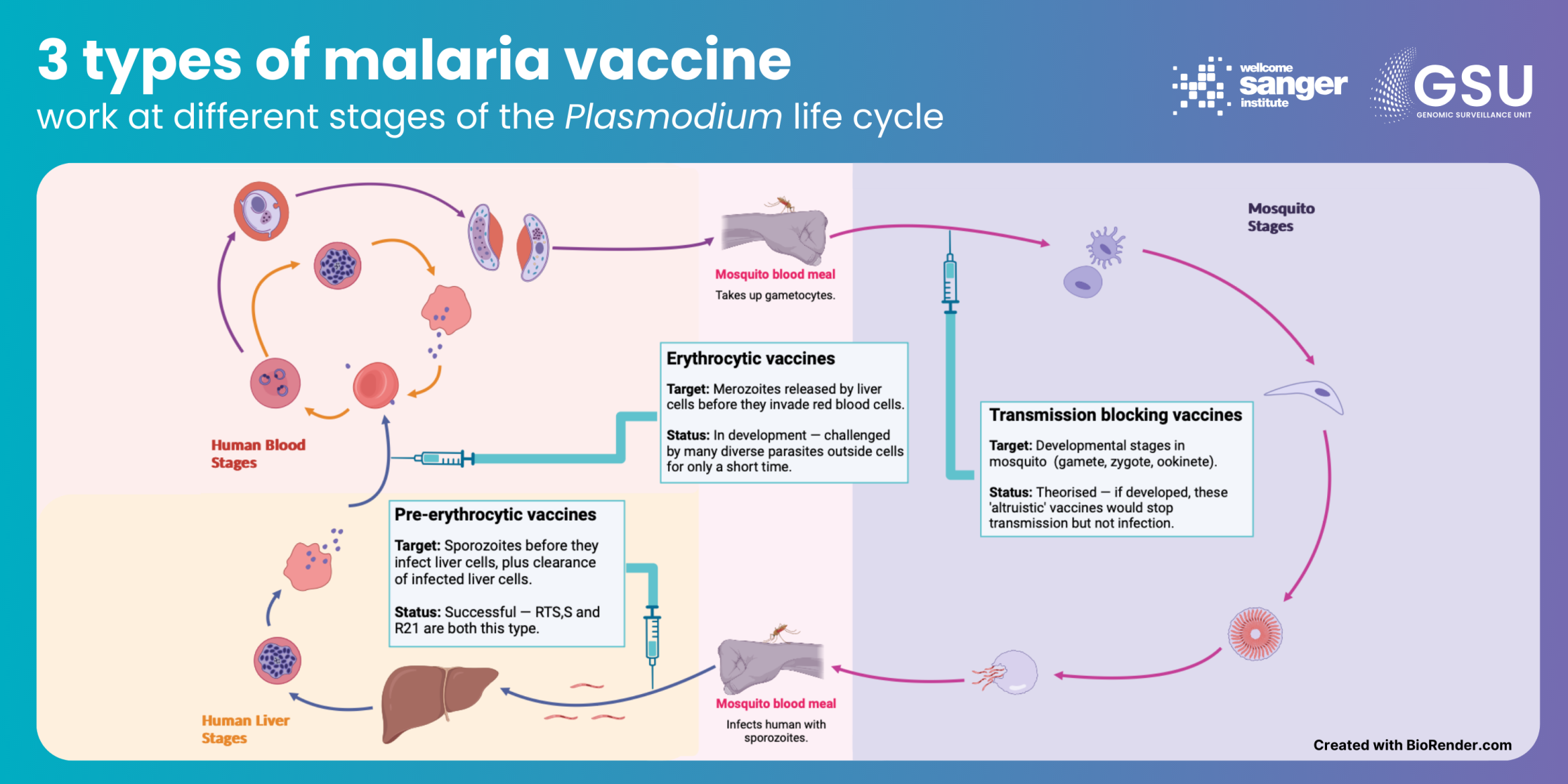
How genomic surveillance can help: Both vaccines work in a similar way. They target sporozoites, the stage in the Plasmodium life cycle that initially infects humans, by introducing parts of the parasites’ circumsporozoite protein (CSP). This encourages a heightened immune response. This is working well so far, with the R21 vaccine shown to reduce symptomatic malaria cases by 75 per cent in the 12 months after a three-dose regimen is given. But we simply cannot know how long that will be the case – the gene that codes for CSP in the malaria parasite is fairly variable, and the parasite may evolve under pressure.
Meanwhile, the data generated for genomic surveillance of malaria can be used to inform development of new vaccines targeting other stages of the Plasmodium life cycle. What’s more, the WHO has stressed that vaccines are in addition to, rather than a replacement of, existing control measures. As outlined in the examples above, genomic surveillance will be important in any future multi-pronged approach against malaria.